The growing role of patient groups in healthcare research
February 2024:
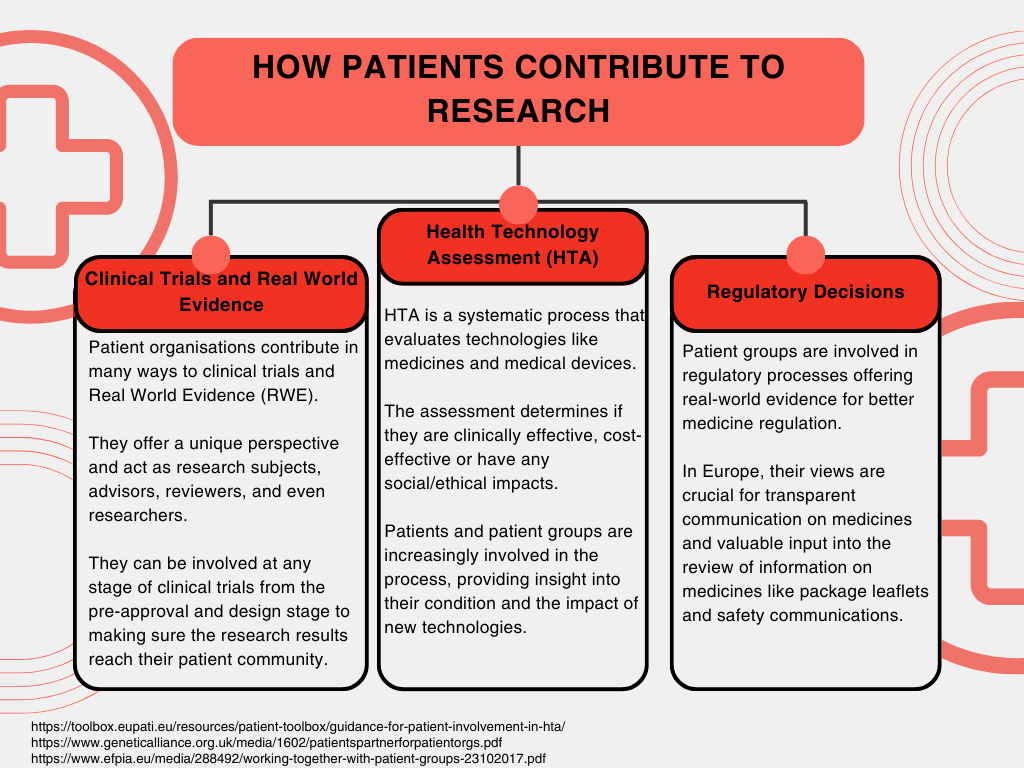
Patient groups have a wealth of experience in the diseases and conditions in which they specialise—at least from a patient perspective. From this standpoint, these groups have traditionally worked to empower patients, raise awareness of medical conditions, and (working alongside other significant healthcare stakeholders) try to ensure that the medical needs of patients are met. More recently, though, patient groups have become integral contributors to health and medical research. They not only provide precise and exclusive insight into the challenges of living with a disease—but deliver valuable observations on how care should be delivered, how it impacts patients, and which therapies can best address patients’ unmet needs [1]. But patient groups have shown their ambitions reach well beyond the above: be it contributing to the design of clinical trials or having the patient perspectives addressed in health-technology assessments (HTAs), and regulatory decisions.
The concept of ‘patient and public involvement’ (PPI) in research—in other words, research that is conducted with, or by, patients—has become a central aspect of health research, and is widely recognised as “beneficial from a scientific and ethical standpoint[3]”. Large research organisations, including the National Institute for Health Care and Research (NIHR) in the UK, and regulatory bodies, including the Food and Drug Administration (FDA) in the US, are just two examples of healthcare stakeholders which now emphasise the importance of patient input into health research.
In this blog, we’ve highlighted the transformative impact that three very different patient groups have had in the clinical field: the Health Research Charities Ireland (HRCI), the Michael J. Fox Foundation for Parkinson’s Research (MJFF), and A Twist of Fate-ATS (ATOF).
Enhancing the environment for health research in Ireland
Health Research Charities Ireland (HRCI) is a national organisation that brings together charities engaged in health research, medical research, and social-care research. It is a leader in ‘patient and public involvement’ (PPI) in Ireland, helping member organisations to improve the lives of patients through impactful research. A key initiative HRCI funds in collaboration with the Health Research Board (HRB) is the ‘Joint Funding Scheme’. This is a unique programme which allows HRCI members to pursue research efforts meaningful to their community of patients, and yet still manage to adhere to international best practices in research governance. To date, 153 awards have been made under the Joint Funding Scheme, representing an investment of €25 million, to over 27 charities (one being Cystic Fibrosis Ireland, a patient group that works towards the development of treatments for CF). Recently, Cystic Fibrosis Ireland participated in a successful project led by the University of Limerick, aiming to explore the quality of the diet of people with cystic fibrosis and to identify the barriers that people with cystic fibrosis face when trying to eat a healthy diet[4].
Supporting ‘breakthrough’ Parkinson’s research
The Michael J Fox Foundation for Parkinson’s Research (MJFF) is dedicated to driving the development of therapies for people living with Parkinson’s, and it does so by funding research into the condition[5]. The Foundation pays for, or sponsors, clinical trials in partnership with academic (and other healthcare) stakeholders, and currently has 15 disease-modifying interventions in trials. The MJFF-sponsored ‘Parkinson’s Progression Markers Initiative’ (PPMI) is said to be “the most-robust dataset and biosample library in the history of Parkinson’s research”[6]. The Initiative has collected brain-scan and biosample-analysis data from 1,500 volunteers. Researchers turn to the PPMI to find facts useful to their work and have downloaded information over 1.7 million times since 2010.
In 2023, PPMI staff achieved a breakthrough, discovering a biomarker capable of detecting a protein known as ‘Parkinson’s protein’ (a fundamental element of the pathology of the disease). The biomarker tool can discern, with 93% accuracy, the presence of this protein in the spinal fluid of individuals at high risk of developing Parkinson’s (but who have not yet been diagnosed with Parkinson’s, or even shown any clinical symptoms)[7]. The discovery stands as a milestone on the road to a cure for Parkinson’s disease, and may never have come about without the backing of patient groups like the MJFF.
Empowering patients with rare connective-tissue disorders
International patient group A Twist of Fate-ATS (ATOF) searches for a cure for arterial-tortuosity syndrome (ATS). ATOF also provides resources and support for patients with connective-tissue disorders (and their families). As part of its mission, ATOF established in 2022 a forum entitled CONECT (Cardio-Ocular Network ConnEctive Tissue), designed to allow patients with other, rare, connective-tissue disorders (such as Marfan syndrome, and cutis laxa), and the patient groups that specialise in these conditions, to engage with clinicians and medical experts—all with the goal of helping to assess treatment options and to make informed decisions about patient care. Patients share with clinicians their experiences via discussions, panels, surveys, and meetings. The results can then form the basis of an educational platform (to include webinars and patient hotlines), for multi-stakeholder engagement on ATS, plus other related conditions.
Patient engagement is essential in the healthcare industry offering unique insights into how patients and their families navigate their conditions and increasingly impacts decision-making. The data collected from patient engagement, Patient Engagement Data (PED), plays a critical role in health research ensuring that healthcare stakeholders can deliver evidence-based impact enhancing health outcomes and meeting patient needs seamlessly[8].
In its current survey of patient groups, PatientView explores patient engagement in pharma’s R&D.
This is an invitation to patient groups to participate in PatientView’s ‘Corporate Reputation of Pharma’ survey offering them the opportunity to share their opinions on whether the pharmaceutical industry has met the needs and expectations of patients and patient groups during the last year—including the industry’s engagement of patients and patient groups in their R&D.
This year’s survey is available in 23 languages. If you wish to take part and share your views on how the pharma industry should work at improving healthcare outcomes for patients, please fill out the questionnaire (to be found by clicking here).
[1] https://www.efpia.eu/media/288492/working-together-with-patient-groups-23102017.pdf
[2] https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7075437/
[3] https://www.cfireland.ie/research/current-projects/exploring-diet-quality-in-cystic-fibrosis
[4] https://www.michaeljfox.org/our-promise
[5] https://www.michaeljfox.org/our-impact
[6] https://www.michaeljfox.org/news/breaking-news-parkinsons-disease-biomarker-found
